In a recent post, I provided the first ever glimpse of the previously unknown larva of Cylindera celeripes, or swift tiger beetle. This little-known flightless species is among the tiniest in North America (adults measure only 8 or 9 mm in length), and so far nobody has succeeded in rearing the species in the lab, or even finding its larva. As the photographs in that post showed, I am reasonably close to accomplishing that first goal, having successfully obtained a number of eggs from field-collected adults placed in a terrarium of native soil. I fed the subsequent larvae a diet of small rootworm larvae and Lygus nymphs before putting them to sleep for the winter in a cold incubator, and the larvae resumed activity when I pulled them out of the incubator 2 months ago. Since then, they have feasted heavily on small noctuid larvae that we rear in our lab, and now most of the dozen or so larvae have sealed their burrows – I presume for pupation before (hopefully) emerging as adults in the next few weeks.
There is more to the story, however. I had brought the adults back home in June 2009 from a population I found at Alabaster Caverns State Park in northwestern Oklahoma. This was a reasonably robust population – news enough for a species that has not been seen in good numbers for many years now, and my discovery of equally healthy populations at several other locations in the general area gives new hope for the long-term prospects of a species that some regard as a potential candidate for listing as an endangered species. It also gave me hope that I might be able to find the larva were I to return to the area in the fall. I also had a hunch that Cicindela pulchra (beautiful tiger beetle) could be found in the area, based on some very large larvae I found during that June trip, so in early October I made a quick return to northwestern Oklahoma to search for these two species. While it was too cold and wet to have any hope of finding Cicindela pulchra adults (I still think the species is there), it did not prevent me from realizing my other goal. May I present one of the first ever field-collected larvae of Cylindera celeripes!

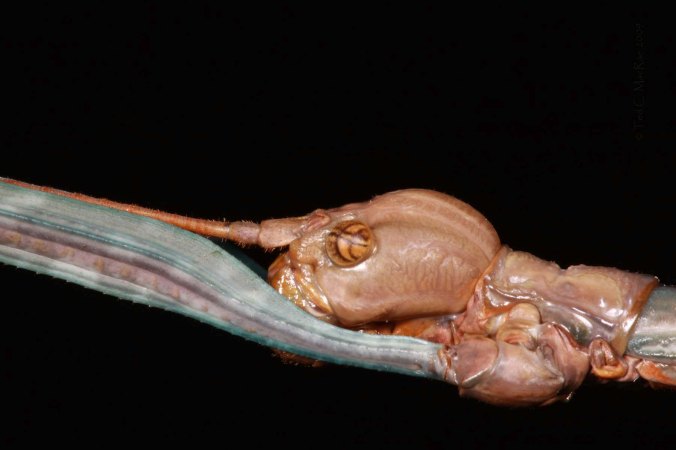
Cylindera celeripes 3rd instar larva - closeup of hump on 5th abdominal segment with hooks to aid in securing the larva in its burrow
I found the larvae at Alabaster Caverns where I had found the adults earlier in June, and although the larval burrows were very small (only 2 to 3 mm in diameter), I knew what they were immediately when I saw them. As I had observed for the adults, burrows tended to be near the edges of barren patches of soil in proximity to vegetation and not out in the middle of the barren areas. This makes sense, considering where it would be more likely for prey to be encountered. Because the weather was cold and gray, I didn’t see (or expect to see) larvae actively sitting at the tops of their burrows, so I began “fishing” to see if I could yank a few from their burrows. I fished quite a few burrows for the first half hour or so, but none of my attempts were successful. I began wondering if the larvae were even active at all or if they had already entered hibernation for the upcoming winter. While I was fishing, I noticed that the burrows all seemed rather shallow – only about 6” or so (most tiger beetles, having larger larvae, dig burrows that are much deeper). This gave me an idea. I went back to the truck and retrieved a small spade that I carry in case… well, I’d never actually used it before. Anyway, I inserted a grass stem into a burrow and sunk the spade into the ground right next to it, making sure I got the spade at least as deep as the grass blade. I then removed the spade and sunk it into the ground on the other side of the burrow, then pried until the entire chunk of soil came up intact. With the bottom of the soil chunk exposed, I used my knife to carefully remove slivers of soil until I found the end of the grass stem that I had inserted into the burrow. Carefully removing the soil in this area revealed the larva in a side chamber at the bottom of the burrow. Success! I took many photos of that larva right then and there, and over the next hour or so collected several more larvae, all but one of which I presumed were 3rd instars. I packed each larva in its own small vial of native soil for the trip home, and although I have been attempting to rear them out for confirmation of their identity, there is little doubt that they do indeed represent this species.
The photographs I’m showing here are not those first field photographs that I took when I first discovered the larvae. Looking at those photographs after I returned home, I was dissatisfied with the amount of soil and debris that covered the larvae – especially their grotesquely unique head and pronotum. Instead, I removed one of the larvae from its rearing tube and gave it a “bath” – brushing it with a fine camel-hair brush in a shallow dish of water – to clean it up for the photographs shown here. After the photo shoot, I sacrificed this larva for the collection – it will be the basis for a formal description of the larva of this species (along with examples of the 1st and 2nd instars that I had sacrificed from my rearing, not yet confident that I would succeed in getting any of the others to 3rd instar). The only thing I am waiting on before preparing that description is to see whether I actually succeed in rearing this species from egg to adult – stay tuned!
Photo Details: Canon 50D (ISO 100, 1/250 sec, f/13-16). Canon MP-E 65mm 1-5X macro lens, MT-24EX flash (1/8 power) w/ Sto-Fen diffusers. Typical post-processing (levels, unsharp mask).
Copyright © Ted C. MacRae 2010