When I purchased my insect macrophotography rig two summers ago, I already knew that one of the biggest challenges I would face (besides a steep learning curve) was lighting. While it is possible to do insect macrophotography using only natural light, this generally requires the use of a tripod and reflector for all but the largest of insects. Unfortunately, such devices aren’t very practical for field photographs of the tiger beetles that I have come to enjoy stalking (and I already have enough to carry as it is without adding such incumbrances). Supplemental flash lighting is a more attractive alternative for me – not only does it minimize the amount of equipment I must carry, but the high shutter speeds and small apertures it allows are perfect for ‘freezing’ subjects prone to quick movements while maintaining good depth-of-field. There are many flash units to choose from, but I went with the Canon MT-24EX Macro Twin Lite Flash for its dual light sources (eliminating the “flatness” of a traditional ring flash) and lightweight, front-of-lens mounted bracket (no need for heavy bracket extenders). Combined with Canon’s 100mm f/2.8 (up to 1X magnification) or MP-E 65mm (1-5X magnification) macro lens, this flash unit has become quite popular in recent years for insect macrophotography.
The problem with flash, of course, is the harsh, unnatural light that it produces. With natural lighting, illumination comes from all directions, while with flash it is essentially unidirectional. This is especially problematic with beetles, many of which have a smooth, shiny integument that reflects the flash to produce strong specular highlights. Diffusion and maximizing the apparent size of the light source are key to achieving good results with flash units, and a variety of diffusers are commonly used to achieve this. Unfortunately, the small size of the MT-24EX flash heads and their placement at the front of the lens creates some unique challenges for diffusing their light. The only commercially available diffusers for the MT-24EX (that I’m aware of) are the Stofen OM-24XSET, which are translucent plastic caps that fit over the unit heads. I used these during my first season of photography, and while better than nothing they still leave much to be desired. The problem is that they do nothing to increase the apparent size of the light source, and it is an even worse problem with the 100mm lens than the 65mm because of its longer working distance. Much better results have been achieved by Kurt (Up Close with Nature) with his concave foam diffuser and Alex (Myrmecos) with his tracing paper diffuser. Unfortunately, these diffusers only work with short focal length lenses such as the 65mm, while it is the 100mm lens that I use most often for tiger beetles (1.0-1.5X range). For most of this past season, I tried a Gary Fong Puffer + Sto-Fen combo diffuser based on an idea by Dalantech, but again that setup seemed only slightly better than Sto-Fens alone with the 100mm. As the season progressed, I continued to mull over various contraptions and ideas to extend the flash heads out in front of the lens to increase apparent light size. Most of those ideas were expensive and bulky, but at the end of the season I came up with an idea that seemed like it might work and went with it. The following photographs are the first iteration of that idea.

Canon MT-24EX flash and 100mm macro lens with DIY oversized concave diffuser.
The diffuser is a larger version of Kurt’s do-it-yourself (DIY) concave diffuser. It uses thick polypropylene foam (used as padding in cardboard shipping boxes) that is sturdy enough to hold its shape but flexible enough to curl back and over the top of the flash heads, essentially forming a large “soft box” in front of both flash heads.

Diffuser is ultralight and lays almost flat when not installed - easily carried in camera backpack.
I cut the bottom inch off of a a 1,000-mL polypropylene beaker (the prototype used a 500-mL beaker, but that was too small). I then cut the center out of the beaker bottom so that the hole size matched the lens opening of the flash head bracket, and then cut the beaker bottom in half. This forms a sturdy but translucent, semi-circular frame to hold the polypropylene foam against the flash head bracket on the front of the lens. The piece of foam measures 21″ (front) x 7″ (back) x 9″ (front to back) and is attached to the polypropylene frame using hot glue.

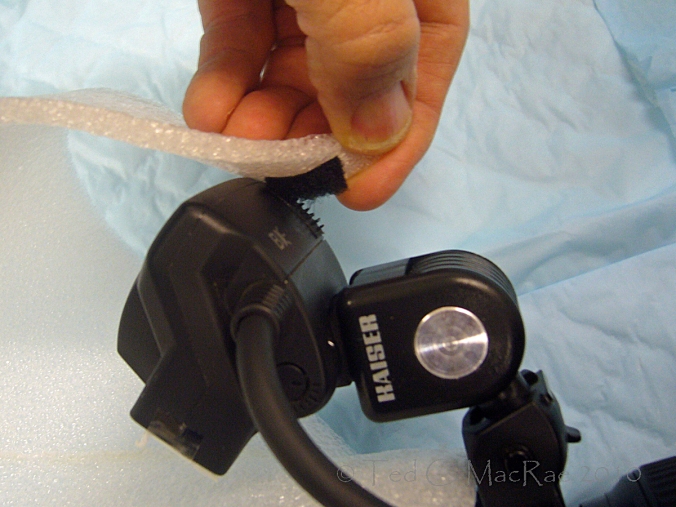
Flash heads extended forward w/ Kaiser shoes. Thin polypropylene foam layer taped over flash head.
I also used Kaiser shoes to extend the flash heads a little further forward in front of the lens, and I taped a small piece of thin polypropylene foam over front of each flash head to provide some initial diffusion. This helps to increase the apparent light size by reducing the distance between the flash heads and the subject. I snugged the pivot screw on the Kaiser shoes just enough to hold the flash head in place but still allow me to adjust their aim.

Diffuser attaches to front of flash bracket using Velcro strips.
The diffuser frame is attached to the front of the flash head bracket using pieces of Velcro strips. It’s not a tight, sturdy connection, but so far I have not had any problems with the diffuser falling off. This system allows me to quickly and easily switch out similar diffusers of different sizes (I have a smaller one that I made for the 65mm lens).

Diffuser attached to flash bracket.
Diffuser is curled back and corners attached to back of flash heads using Velcro.
Pieces of Velcro strip are also attached the corners of the diffuser and the back of the flash heads to hold the diffuser foam in position after attaching the bracket to the flash head bracket.

Diffuser in position and ready for use.

Diffuser remains properly positioned regardless of flash head position or lens changes.
I have since added additional Velcro strips along the front edge of the foam to allow it to be pulled back closer to the flash heads, depending on the distance to subject.
One nice thing about this diffuser is that it does also work with the 65mm lens as long as there is nothing to get in the way of the diffuser. It is simply a matter of angling the flash heads back closer to the lens and adjusting their aim according to the subject distance, then pulling the foam layer back closer toward them. Or, just swap out with a smaller version. When detached, the diffuser can be folded to lay flat in the backpack.
Of course, the proof is in the pudding, and none of this means anything if it doesn’t actually do the job. I’m now immersed in the depths of a Midwestern winter, so I haven’t yet had a chance to test the diffuser in the field. I have, however, done a fair amount of testing here in the laboratory using both live and dead insects and have been quite pleased with the results so far. Those photographs can be seen in the following posts. This coming season I’ll put it to the test in the field to see if it actually has the usability and durability that I have hoping for.
Copyright © Ted C. MacRae 2010






























